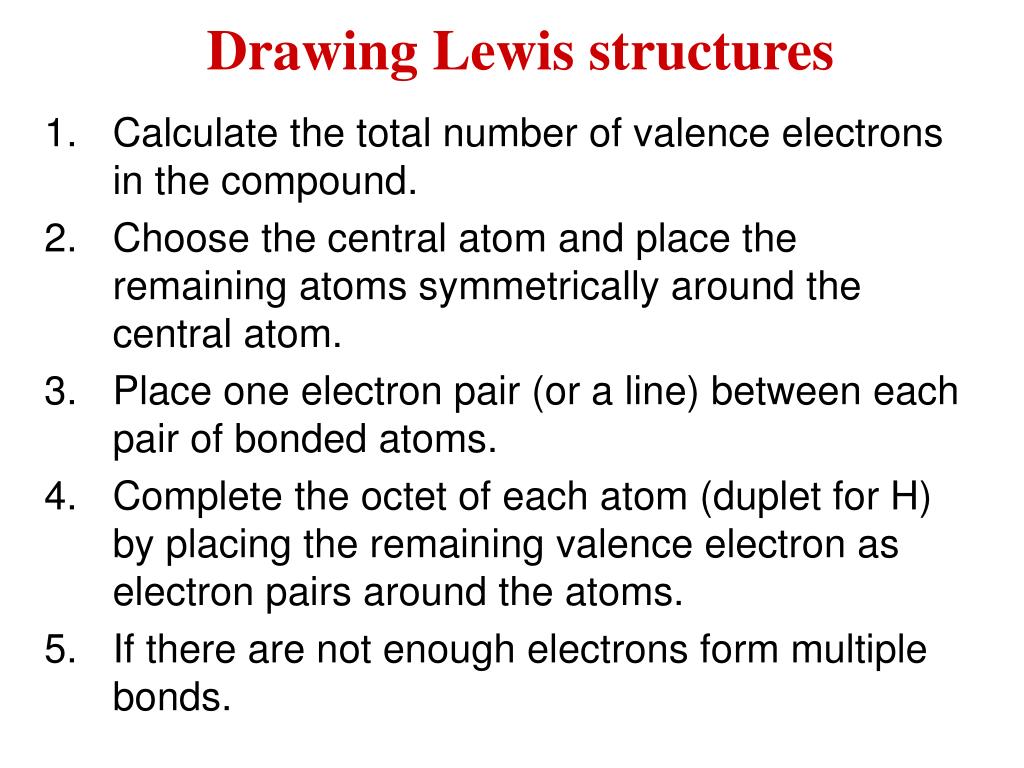
Write the skeletal structure. One way to do this is to write the Lewis symbols for all of the atoms in the formula and count up all the dots.
Ppt Drawing Lewis Structures Powerpoint Presentation Free Download Id 529421
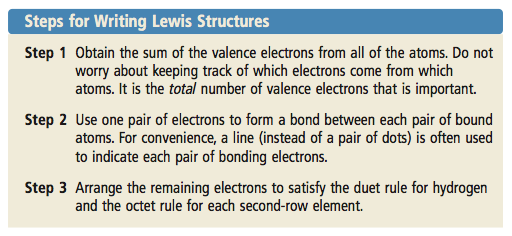
Place one electron pair between each pair of adjacent atoms as determined from the framework found in step 2 to form a single bond.

. Place least electronegative element in center and draw single bonds from the central atom to other atoms. First of all a correct count of all valence electrons is essential. Determine the total number of valence electrons in the molecule or ion.
If there are multiple elements that have more dots than the rest then just pick one at random among the higher dotted elements. Odd number of electrons. Complete pairing of these e- is impossible and an octet around each atom cannot be achieved resonance structures 2.
A H can have only 2 e- 1 bond b Be prefer to have only 4 e- 2 bonds c B and Al prefer to have only 6 e- 3 bonds. Group number for each element of valence electrons. Refer to the first picture b find the element s that has more dots than the rest.
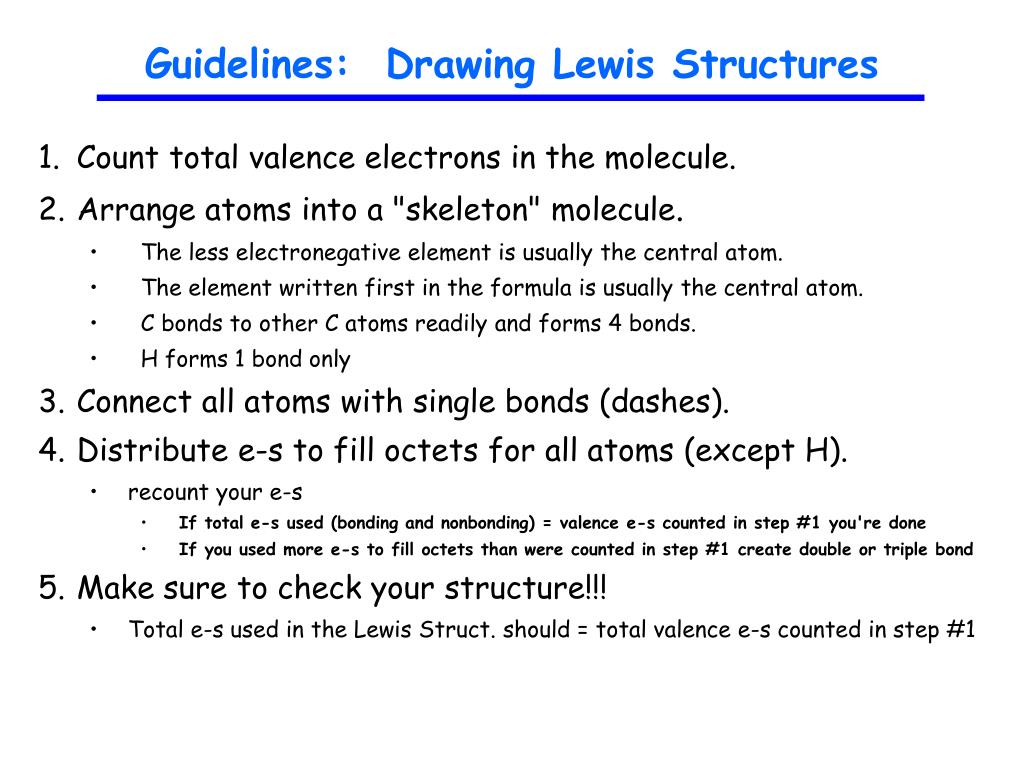
LEWIS STRUCTURES General Rules for Drawing Lewis Structures 1. Count the total number of valence electrons. Draw the initial skeletal structure with atoms connected by single bonds.
How to Draw a Lewis Dot Structure Step 1. Decide on a skeletal structure What is bonded to what The central atom is generally written first in the formula Hydrogen is never the central atom even if it is. For ions the charge must be taken into account.
Less than an octet of valence electrons. Lewis structures can be drawn with a set of general rules that give us a pretty good idea of how valence electrons are distributed around a molecule. Occurs when there are fewer than eight valence electrons around an atom resonance structures 3.
Determine the total number of valence electrons to be depicted in the Lewis diagram. For cations subtract a number of electrons equal to the positive charge. The ultimate goal is that all atoms end up with 8 e-s.
If the charge is negative add electrons and if the charge is positive subtract electrons Step 2. Rules for drawing Lewis dot structures Count the number of valence e- each atom brings into the molecule. Rules for Drawing Lewis Structures For molecules known to exist follow these rules to determine the Lewis structure.
The following rules are given to assist you. General Rules for Drawing Covalently Bonded Lewis Structures 1. MUST FOLLOW IN ORDER.
Rules for Drawing Lewis Structure. It should be the most symmetric structure you can draw. Put electron pairs about each atom such that there are 8 electrons around each atom octet rule with the exception of H which is only surrounded by 2 electrons.
-Hydrogen cannot be central. Determine the central atom and write the skeleton structure of the molecule. You are expected to be able to draw such structures to represent the electronic structure of compounds.
Sometimes the number of valence e- is odd. Rules for drawing Lewis structures. Place electron pairs around each terminal atom except H to complete octets.
For the most part Lewis structures reflect what is found when we gather experimental data for molecules in the laboratory. For a molecule uncharged that count is the correct. Add electrons for negatively charged ions.
This is a must. All atoms want to fulfill the octet rule. Unpaired electrons are observed in odd electron molecules such as NO and NO2.
For ions the charge must be taken into account. The only exceptions are. Determine whether the compound is covalent or ionic.
Rules for drawing Lewis dot structures. Refer to the second picture. Subtract electrons for positively charged ions.
Instructions for Drawing Lewis Structures For molecules and polyatomic ions composed of nonmetals. Rules for drawing Lewis dot structures Count the number of valence e- each atom brings into the molecule. Put electron pairs about each atom such that there are 8 electrons around each atom.
All valence electrons of the atoms in Lewis structures must be shown. The central atom most of the time is the more positive atom. Determine how many electrons must be added to central element.
Count the total number of valence electrons in the structure Remember Group of Valence electrons 2. Given a chemical formula corresponding to a molecule or molecular ion draw a Lewis structure. Its not perfect but it is a useful tool for scientists trying to understand how molecules will react and their.
A Draw the lewis structure by repeating steps 1-4. Hydrogen 2 valence Boron 6 valence Aluminum 6 valence Gallium 6 valence Indium 6 valence Sometimes Exceptions. Generally electrons are paired.
The least electronegative atom will be central. -Atoms that occur once or first are usually central. Make sure that H has only 1 bond and that the other atoms do not exceed their normal number 2 bonds to O 3 bonds to N 4 bonds to C and that no atom in the molecule has more than 4 bonds.
1 hour agoWhen drawing Lewis Dot Structures for ionic compounds you need to follow a different set of rules than with Lewis Structures for covalentmolecular compounds I can draw a Bohr diagram that shows what happens in an ionic bond between two elements I can count the number of atoms in an ionic compound using its chemical formula I can write the. General rules for writing LEWIS structures for molecules. RULES FOR LEWIS STRUCTURES A Lewis structure consists of the electron distribution in a compound and the formal charge on each atom.
CBr 4 -The least electronegative atom is central. CO 2 Total 16 Step 2. Nitrogen 5 valence Phosphorus 10 valence.

I Can 2 I Can Draw A Lewis Structure Rules For Lewis Structures 1 Total Number Of Valance Electrons 2 Central Atom Always C Never H Rarely O Or Ppt Download

Electron Dot Model Of Bonding Lewis Structures
Lewis Electron Dot Diagrams Help

Lewis Structure Organic Chemistry Video Clutch Prep
Ppt Guidelines Drawing Lewis Structures Powerpoint Presentation Free Download Id 1812949


0 comments
Post a Comment